Describe the Common Ion Effect and Give an Example
Explain why this is an unusual property and give an example of how this is important to living things. Regardless of what is added to water however the product of the concentrations of these ions at equilibrium is always 10 x 10-14 at 25 o C.
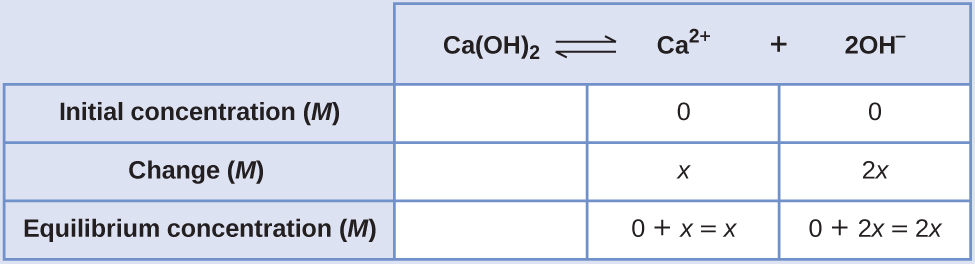
15 1 Precipitation And Dissolution Chemistry
H 3 O OH- 10 x 10-14.
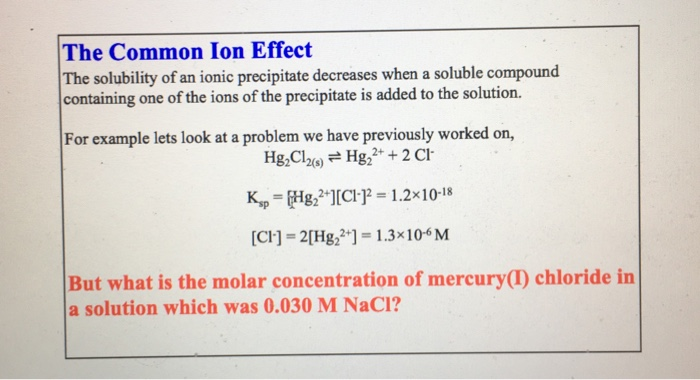
. Describe how water is used as the basis of the scientific measurements of temperature mass and energy. Adding an acid to water increases the H 3 O ion concentration and decreases the OH-ion concentration. Adding a base does the opposite.
Describe an aqueous solution. The mechanism was discussed in Section 52 and details about the mobile phases used for ion pairing were given in Section 77Ion pairing is a. The table below lists pairs of H 3 O and OH-ion.
Moldoveanu Victor David in Essentials in Modern HPLC Separations 2013 96 Separations by Ion-Pair Chromatography General Comments. Various aspects related to ion-pair chromatography were presented previously. Ion-exchange chromatography which is designed specifically for the separation of differently charged or ionizable compounds comprises from mobile and stationary phases similar to other forms of column based liquid chromatography techniques 9-11Mobil phases consist an aqueous buffer system into which the mixture to be.

Molar Solubility Example Calculation Common Ion Youtube

Solved The Common Ion Effect The Solubility Of An Ionic Chegg Com
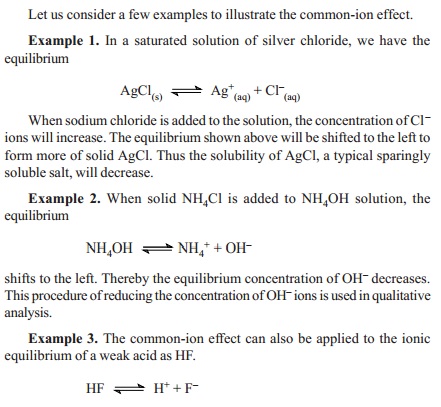
Kohlraush S Law Application With Example

Common Ion Effect And Buffers Video Khan Academy

Equilibrium Class 11 Notes Chemistry Chapter 7 Learn Cbse

Effect Of A Common Ion On Solubility Introduction To Chemistry

Solubility Product Ksp Definition Formula Significance Faqs

What Is Common Ion Effect Chemistry Chimica
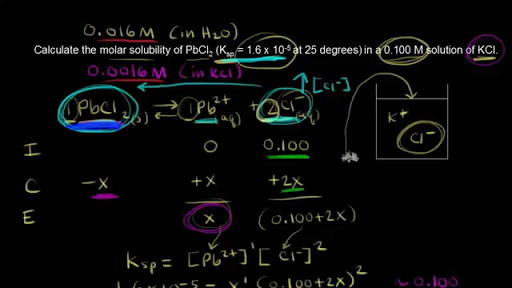
The Common Ion Effect Video Equilibrium Khan Academy

Neet Ug Common Ion Effect Offered By Unacademy
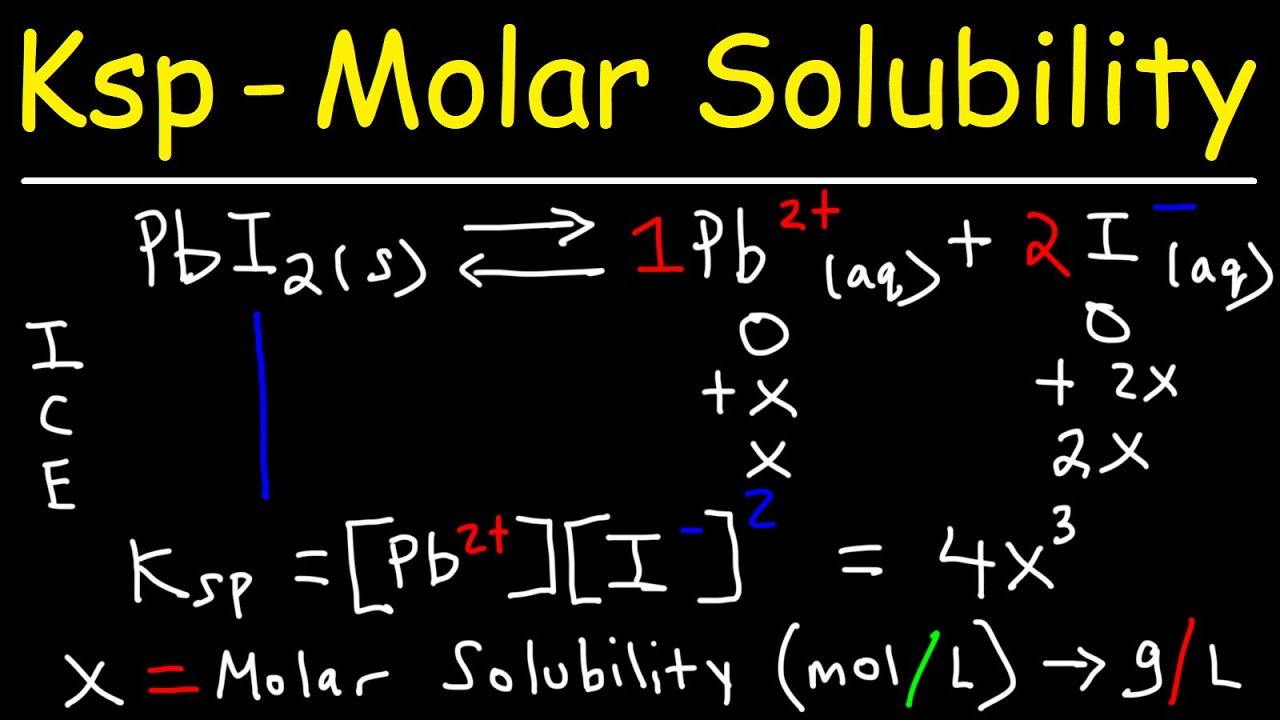
Ksp Molar Solubility Ice Tables Common Ion Effect Youtube

Sch4u Acids And Bases Buffer Solutions Ppt Download

The Common Ion Effect Video Equilibrium Khan Academy

Common Ion Effect Statement Explanation And Examples
18 3 Common Ion Effect In Solubility Equilibria Chemistry Libretexts



Comments
Post a Comment